Dr. Sai Sudha Koka’s Lab
Research Goals
The Koka lab focuses on three areas:
- Etiology of cardiovascular diseases
- Developing novel therapeutic strategies for prevention and treatment of cardiovascular diseases in diabetic, obese and aging patients.
- Cardiotoxicity of novel anti-cancer therapies
Cardiovascular disease pathogenesis: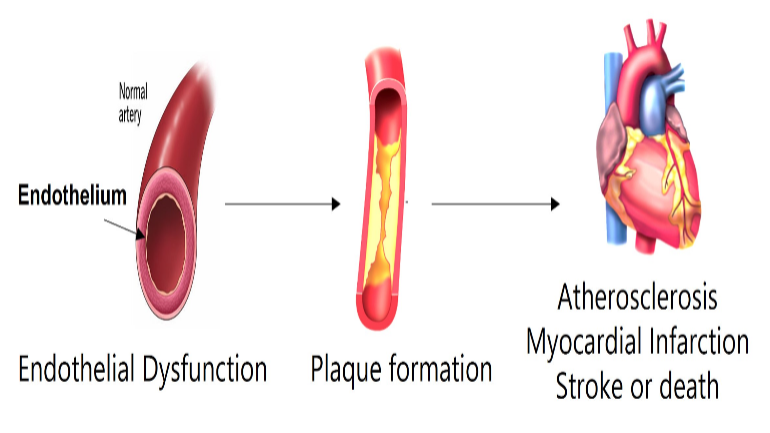 Cardiovascular diseases (CVD) represent the leading cause of morbidity and mortality in the world. Our current understanding of the CVD pathogenesis is insufficient to control serious cardiovascular complications in majority of the patients who eventually succumb to the disease. Hence, there is a critical need for discovery of novel risk factors and pathways linked to CVD pathogenesis. In this context, the role of gut microbiome and gut microbe-derived metabolites on vascular disease pathogenesis has been recognized only in recent times. Our lab focuses on studying the molecular mechanisms mediating the response of endothelial and vascular smooth muscle cells to gut microbe derived metabolites. We discovered that gut microbial metabolites like trimethylamine-N-oxide (TMAO) induce inflammasome activation leading to endothelial dysfunction, vascular injury and atherosclerosis in mouse models.
Cardiovascular diseases (CVD) represent the leading cause of morbidity and mortality in the world. Our current understanding of the CVD pathogenesis is insufficient to control serious cardiovascular complications in majority of the patients who eventually succumb to the disease. Hence, there is a critical need for discovery of novel risk factors and pathways linked to CVD pathogenesis. In this context, the role of gut microbiome and gut microbe-derived metabolites on vascular disease pathogenesis has been recognized only in recent times. Our lab focuses on studying the molecular mechanisms mediating the response of endothelial and vascular smooth muscle cells to gut microbe derived metabolites. We discovered that gut microbial metabolites like trimethylamine-N-oxide (TMAO) induce inflammasome activation leading to endothelial dysfunction, vascular injury and atherosclerosis in mouse models.
Pre-clinical development of small molecule inhibitors for treatment of CVD:
Despite maximal therapeutic intervention with currently available treatment options, at least 50% residual risk remains in CVD patients. There is ongoing search for new drugs as well as repurposing FDA approved drugs for the treatment of CVD. We are currently focusing on investigating the therapeutic potential of novel small molecules which include inflammasome inhibitors, nanoparticle-based formulations and natural products in ex-vivo and in vivo models of CVD.
Drug-induced Cardiotoxicity:
Several chemotherapeutic agents like anthracyclines are known to be cardiotoxic. Despite their efficacy, their dose-dependent cardiotoxic side effects have been a major clinical concern that limits their clinical usage. The cardiotoxic effects of the most recent and novel chemotherapeutic agents are unknown. We have earlier demonstrated that phosphodiesterase-5 (PDE-5) inhibitor tadalafil attenuated cardiac dysfunction in doxorubicin-induced cardiomyopathy. Our lab focuses on identifying the cardiotoxic effects of novel agents and studying the pathogenesis of chemotherapeutic drug-induced cardiotoxicity.
Lab Funding
- R01HL14711-01A1 Sai Koka (PI); NHLBI, The role of Role of Trimethylamine-N-Oxide in endothelial dysfunction. 08/2022-03/2027.
- R56HL143809 Sai Koka (PI); NIH High Priority, Short Term Project Award, Gut microbial metabolite- Trimethylamine-N-Oxide and endothelial inflammasome signalling in cardiovascular injury. 09/2019-08/2022.
- 19AIREA34380223 Sai Koka (PI); AHA Institutional Research Enhancement Award (AIREA). Role of Trimethylamine-N-Oxide (TMAO) in endothelial and vascular injury 01/2019-12/2021.
Publications
- Kukreja RC, Wang R, Koka S, Das A Samidurai A, Xi L. Treating diabetes with combination of phosphodiesterase 5 inhibitors and hydroxycholorouine-a possible prevention strategy for COVID-19? Mol Cell Biochem. 478(3):678-696, 2023.
- Zhang Y, Wang YT, Koka S, Zhang Y, Hussain T and Li X. Simvastatin improves lysosome function via enhancing lysosome biogenesis in endothelial cells. Front Biosci (landmark Ed). 25:283-298, 2020.
- Koka S, Xi L and Kukreja RC. Chronic inhibition of phosphodiesterase 5 with tadalafil affords cardioprotection in a mouse model of metabolic syndrome: Role of Nitric Oxide. Mol Cell Biochem. 468(1-2):47-58, 2020.
- Singh GB, Zhang Y, Boini KM and Koka S. High Mobility Group Box 1 Mediates TMAO-Induced Endothelial Dysfunction. Int J Mol Sci. 20. Pii E3570, 2019.
- Koka S, Xia M, Li PL and Boini KM. Podocyte NLRP3 inflammasome activation and formation by adipokine Visfatin. Cell Physiol Biochem. 53: 355-365, 2019.
- Koka S, Xia M, Chen Y, Bhat OM, Yuan X, Boini KM and Li PL. Endothelial NLRP3 inflammasome activation and arterial neointima formation associated with acid sphingomyelinase during hypercholesterolemia. Redox Biol. 13:336-344, 2017.
- Boini KM, Hussain T, Li PL and Koka S. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell Physiol Biochem. 44: 152-162, 2017.
- Boini KM, Koka S, Xia M, Gehr TB, Ritter JK and Li PL. Sphingolipids in Obesity and its Complications. Frontiers in Bioscience (Landmark edition). 22: 96-116, 2017.
- Boini KM, Koka S, Xia M, Gehr TW, Li PL. Instigation of NLRP3 Inflammasome Activation and Glomerular Injury in Mice on the High Fat Diet: Role of Acid Sphingomyelinase Gene. Oncotarget, 7 (14): 19031-19044, 2016
- Koka S, Aluri HS, Xi L, Lesnefsky EJ, Kukreja RC. Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1α signaling. Am J Physiol Heart Circ Physiol, 306(11):H1558-68, 2014.
- Das A, Durrant D, Koka S, Salloumn FN, Xi L, Kukreja RC. mTOR Inhibition with Rapamycin Improves Cardiac Function in Type 2 Diabetic Mice: Potential Role of Attenuated Oxidative Stress and Altered Contractile Protein Expression. J Biol Chem. 289(7):4145-60, 2013.
- Koka S, Das A, Salloumn F, Kukreja RC. Phosphodiesterase-5 inhibitor tadalafil attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in Type 2 diabetic mice. Free Radic Biol Med. 60:80-8, 2013.